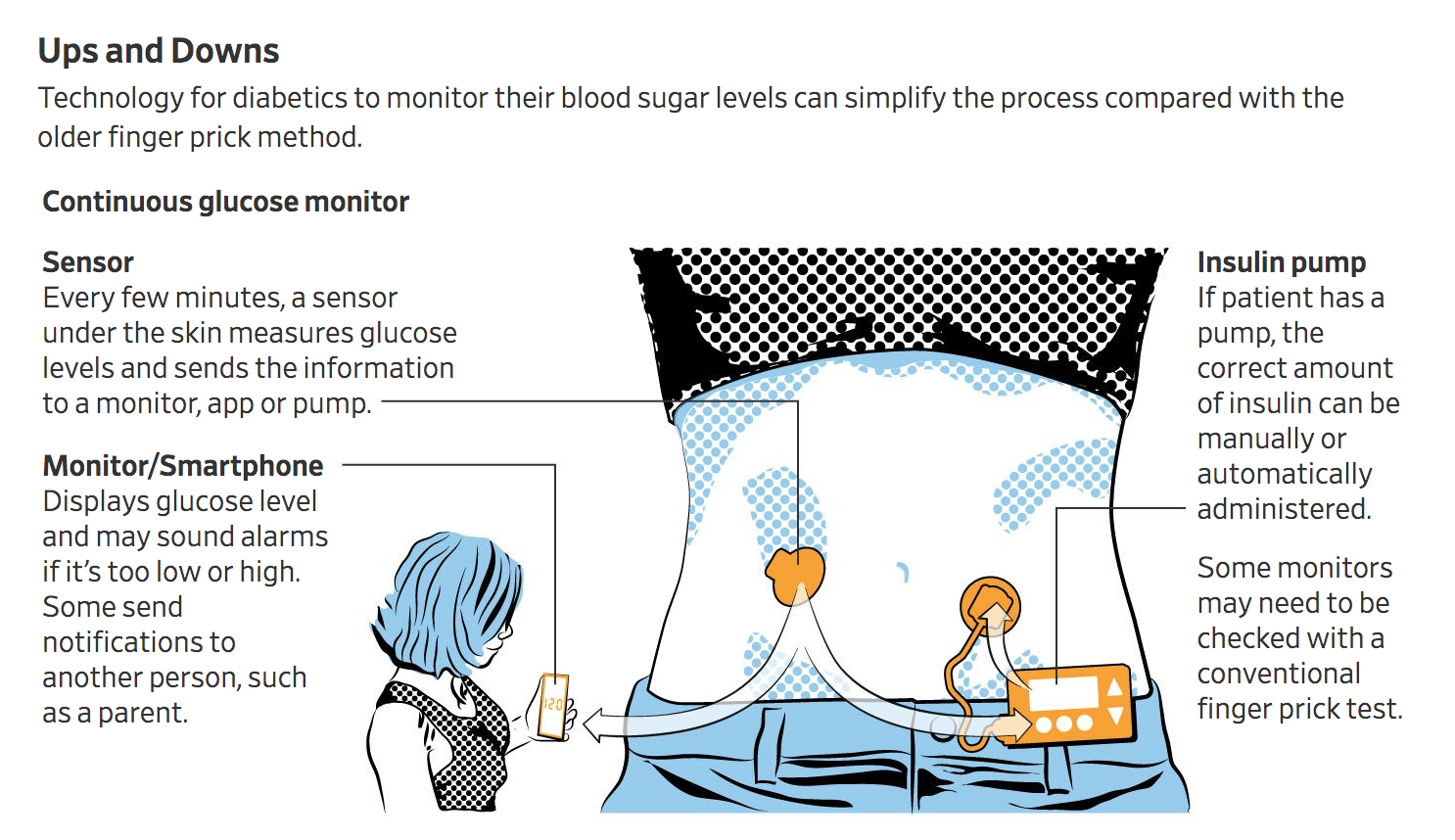
CGMs (continuous glucose monitors) can reduce the stress and uncertainty related to glucose monitoring. Healthy Living is here to help lessen the challenges of acquiring and maintaining a CGM. And it’s easy to get started! Our team will guide you through the whole process.
Here is a preview of what your first conversation with us might include, plus some other details of the progression of obtaining a CGM system.
Intro: Is this your first CGM system? If not, when was the last time you received supplies?
Basic Information: We ask questions to gather patient demographic info, insurance info, and doctor information.
CGM Options: What system are you looking for? We offer Dexcom G5, Dexcom G6, Guardian Connect, or FreeStyle Libre CGM.
Medicare CGM Requirements: Medicare patients must use the Dexcom G5 or FreeStyle Libre system, because Medicare doesn’t cover Medtronic supplies. Must be testing 4x/day and injecting insulin 3x/day.
Authorization & Documentation: If your insurance requires an authorization, the Healthy Living team will gather the appropriate docs, including prescription, chart notes, and 30 days of continuous logs. Once authorization is submitted, you can expect a response time of 7-14 days for most insurance companies, and 30-45 days for straight Medicaid.
Planning for Payment: We will ask for a credit card to place on file to charge once the order is ready to ship. We will never charge you without your permission.
Follow-Up with the Doctor: We will ask you to follow up with your doctor’s office to request required documents for authorization, and we will also reach out to your doctor every 3-4 days until the necessary documents are obtained.
Updates: We will text or call with updates about your account. You will also receive an email with a summary of our conversation and will be given access to our online portal to track the status of the initial CGM order.
Ready for your CGM?
Contact us using your preferred contact method. We have three different ways of helping you - choose whichever is best for you!
1. Call 866.779.8512.
2. Text “CGM” to 248.577.9903.
3. Fill out the form below.